Reductive Amination
NaBH(OAc)3
Examples:
Example 1

A slurry of the amine (50 mg, 0.12 mmol) and the aldehyde (31.6 mg, 0.18 mmol) in 1,4-Dioxane (3 mL) was stirred for 5 min then treated with NaBH(OAc)3 (77 mg, 0.363 mmol). After stirring 10 min, DCM was added to the reaction mixture. The resulting solution was washed with saturated aq NaHCO3, water, and brine. The organics were dried (MgSO4) and concentrated in vacuo to provide the product which was used in the next step without further purification.
[Patent Reference: WO2012129344, page 130, ![]() (7.3 MB)]
(7.3 MB)]
Example 2
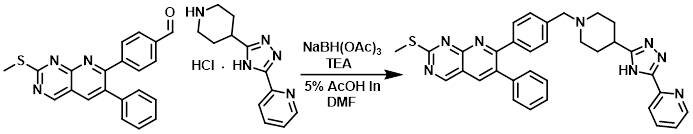
A mixture of the amine (193 mg, 0.727 mmol), aldehyde (200 mg, 0.56 mmol), and TEA (0.074 g, 0.727 mmol) were stirred in 5% AcOH in DMF for 30 min. The reaction mixture was then treated with NaBH(OAc)3 (178 mg, 0.839 mmol) and stirred for 3 h. Upon completion, the mixture was quenched with 3N NaOH, extracted with DCM (3x), dried (Na2SO4), concentrated, and purified by silica gel chromatography (6% MeOH/DCM) to provide the pdt.
[Patent Reference: WO2006036395, page 51, ![]() (4.9 MB)]
(4.9 MB)]
Example 3
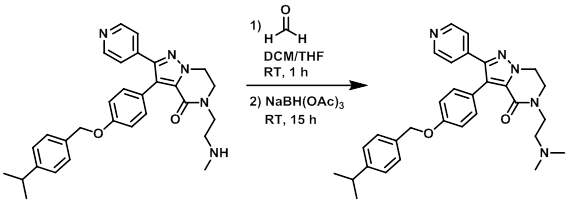
Formaldehyde (46.7 uL, 0.623 mmol) was added to a solution of the SM (100 mg, 0.21 mmol) in DCM (2 mL) and THF (1 mL) at RT. The mixture was stirred for 1 h. NaBH(OAc)3 (88 mg, 0.415 mmol) was added and the reaction mixture was stirred for 15 h. The mixture was poured into aq K2CO3 and was extracted with DCM. The org layer was dried (MgSO4) and concentrated. The resulting material (103 mg) was purified by Prep LC (30 g silica, 1% NH4OH, 69% toluene, 30% IPA) to provide the product. [30 mg, 28%]
[Patent Reference: WO2015144799, page 153, ![]() (18.8 MB)]
(18.8 MB)]
Example 4
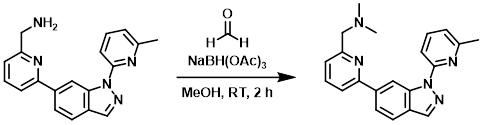
To a mixture of the SM (115 mg, 0.36 mmol) and formaldehyde (aq, excess) in MeOH (10 mL) was added NaBH(OAc)3 (382 mg, 1.80 mmol) at RT. The reaction mixture was stirred at RT for 2 h. After concentration, the residue was purified by prep HPLC to provide the product as a white solid. [60 mg, 48%]
[Patent Reference: WO2016011390, page 65, ![]() (20.2 MB)]
(20.2 MB)]
Example 5
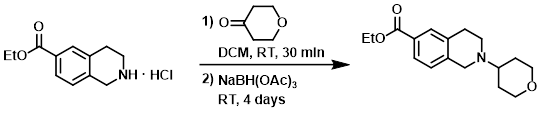
To a solution of the SM (3.45 g, 14.3 mmol) in DCM (150 mL) was added tetrahydro-pyran-4-one (2.0 g, 20 mmol). The mixture was stirred at RT for 30 min, after which time was added NaBH(OAc)3 (12 g, 56 mmol). The reaction mixture was stirred at RT for 4 days, then diluted with sat aq NaHCO3. The layers were separated and the aq layer was further extracted with DCM. The combined organics were dried (Na2SO4) and concentrated. The residue was purified by silica gel flash chromatography to provide the product. [2.51 g, 45%]
[Patent Reference: WO2016014463, page 119, ![]() (6.7 MB)]
(6.7 MB)]
